Coordination Number Ceramics

A 2 and 3.
Coordination number ceramics. The concept is most commonly applied to coordination complexes. The packing factor of bcc is 0 68. D 4 6 and 8. C 6 8 and 12.
The coordination number of the bcc structure is 8. The coordination number of the fcc structure is 12. The cn does not distinguish the geometry of such complexes i e. 4 for r c r a between 0 414 and 0 732 the cation may be thought of as being situated at the center of an octahedron surrounded by anions one at each corner.
The number of nearest neighbors surrounding an ion. Number of spheres in a unit cell. The cation is located at the center of a tetrahedron with anions at each of the four corners. Octahedral vs trigonal prismatic.
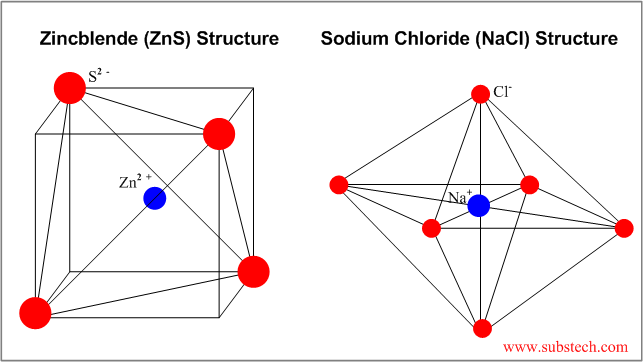
Ceramics chapter 11 ionic arrangements in ionic solids ionic solids cations and anions in the unit cell packing of the ions is determined by. The coordination number is for r c r a between 0 225 and 0 414. A unit cell of bcc has a net total of 2 spheres. 0 00 coordination of hexagonal close packed hcp structure 4 40 theoretical density 21 37 structure of ceramics using cation anion ratio 30 65 nacl rock salt.
In chemistry coordination number c n defined originally in 1893 by alfred werner is the total number of neighbors of a central atom in a molecule or ion. Cerium iv oxide also known as ceric oxide ceric dioxide ceria cerium oxide or cerium dioxide is an oxide of the rare earth metal cerium it is a pale yellow white powder with the chemical formula ceo 2 it is an important commercial product and an intermediate in the purification of the element from the ores. Simple and commonplace cases. Features geometry of ceramics and associat.
Show that the minimum cation to anion radius ratio rc ra for a coordination number of 8 is 0 732. A unit cell of fcc has a net total of 4 spheres. The most common coordination number for d block transition metal complexes is 6. The packing factor of fcc is 0 74.
Materials science problem that calculates the minimum cation to anion radius ratio for a coordination number of 4. The relative size of the ions 2. B 6 and 12. In this case the maximum number of atoms that be coordinated around any individual is 12.
If all of the atoms in a crystal are the same size then there are two ways to pack the atoms to form a crystal structure. Which of the following are the most common coordination numbers for ceramic materials. Material science i exercise 1 structure of ceramics 24 11 2008 1 question 1.